What is Trichoderma spp.?
Trichoderma spp. are genus of fungi commonly found in endophytic plant symbionts, grows along the outermost root surface, and feeds on soil microbes attracted to the root system. This fungus grows with root intercellular spaces, coordinates plant defense system and is widely used to control disease, enhance plant growth, and improve the crop yield (Siemering et al., 2016).
How can Trichoderma spp. help my crops?
Relief from environmental stresses
In case of seed treatment, Trichoderma spp. releases metabolite (gliotoxin) that mimic plant growth hormone, enhances seed generation percentage, activate enzymes and phytohormones property to alter soil microflora for increased nutrient accessibility and uptake-rate in soil. Improve plant morphology and physiology with root enhancement, alter photosynthetic rate during the early stage of plant growth, control the environmental buffering (pH, drought, water-logging, cold and heat) and involved in phosphorus uptake, cellulase release to organic matter decomposition, mineral chelation and siderophore production (Singh et al., 2018).
Biotic and Abiotic conditions
Treated seed, germinate consistently faster and more uniformly in comparison to untreated seeds under biotic stress (seed and seedling disease caused by Pythium ultimum) and abiotic stresses (osmotic, salinity, chilling, or heat stress). The plant–fungus association reduces the accumulation of toxic reactive oxygen species (ROS) and lipid peroxides in seedlings under stress conditions. Improves seedling vigor and ameliorates stress by inducing physiological protection in plants against oxidative damage (Mastouri et al., 2010) (Zin et al., 2016) as represented in figure 1.
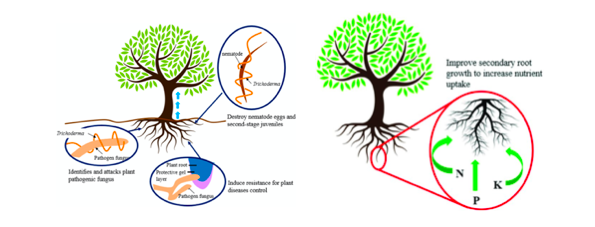
Plants immune system response
Trichoderma-pathogen interactionfollows different mode of action. In case of mycoparasitism involves direct attack on pathogen, which involves recognition of the host: by carbohydrate binding with pathogens, coiling and appressoria formation: induce penetration, secretion of hydrolytic enzymes: fungitoxic cell-wall-degrading enzymes, penetrations of the hyphae: dissolution of the cell walls, and finally lysis of the host: direct entry and killing of the pathogen.
In case of antibiosis mode of action, secondary metabolites, or antibiotics (gliovirin, gliotoxin, viridin, viridol, koninginins, pyrones and peptaibols) produced are detrimental for the growth of pathogens(Singh et al., 2018). These metabolites inhibit the growth of soil-borne plant pathogens, prevents spore germination, and induce extensive apoptotic programmed cell death. During competition mode of action, in the vicinity and molecular assembly of Trichoderma spp. restrict the accessibility of limiting nutrients and starvation of nutrients leads to the death of pathogens. (Singh et al., 2018).
Plant disease management
Trichoderma spp. plays an antagonistic role against the growth of clubroot pathogen (Plasmodiophora brassicae), causes disease reduction (Botero Ramírez et al., 2016), by completely suppressing formation of galls. In addition, enhances photosynthesis with increased chlorophyll activity followed with improved nutrition uptake correlated with higher yields in agricultural and horticultural crops (Suada et al., 2017) (Upadhyay et al., 2020) as represented in figure 2. Similar role against soil-borne fungal pathogens such as Sclerotium rolfsi and Rhizoctonia solani, showed systematic resistance response and reduction in severity of the diseases up to 80% (Debao et al., 1993) (Cheah et al., 2000) (Hanson et al., 2000).
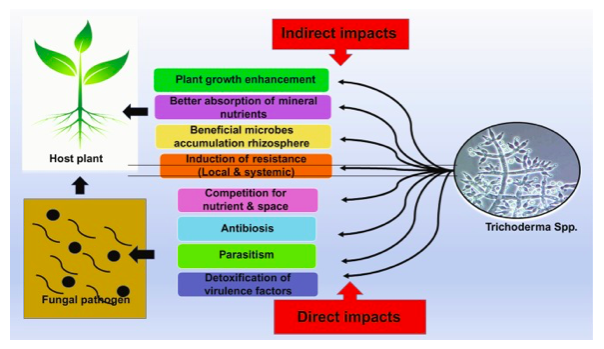
Figure 2: Plant disease management by Trichoderma spp. as represented by (Upadhyay et al., 2020).
Should I inoculate my crops with Trichoderma spp.?
Increased crop yield
Trichoderma spp. account for 60% of the registered bio-fungicides in the market and are the most successful biocontrol agents. Trichoderma spp. possess many qualities such as improved seed germination with plant growth development, increase nutrient uptake rate in plants alter morphological and physiological features, enhance nitrogen-use efficiency among different crops, and assist photosynthetic efficiency(Sood et al., 2020). Guaranteed high yield production in case of crops like mustard, wheat, corn, tuberose, sugarcane, tomato, okra, etc. In legume crops such as lentil and pea, Trichoderma spp. treatment delivered resistance to root, promotion in plant root and increase in shoot biomass was observed (Bazghaleh et al., 2020), showed significant positive effects on seed germination, root length and vigour index in soybean (Živanov et al., 2013), similar effect in rice and potato (Abbas et al., 2017). During growth of pea plants, showed increased percentage of germination rate, increased photosynthetic pigment (chlorophyll), carotenoid, total sugar, and protein content (Singh et al., 2015). In case of lentil, delivered lowest foot and root rot incidence (6.9%), highest seed germination (82.08%), maximum plant stand (93.12%) and the highest seed yield (3726.67 kg ha1) (Kashem et al., 2011) and improved tolerance towards salinity and drought for rapeseed plant (Poveda et al., 2020).
Will my crop management practice impact Trichoderma spp. growth?
Crop management practise such as crop rotations, tillage, seed variety, seeding rate, sanitation, summerfallow, trap strips, soil fertility, intercrops, biological control, pest, and insect management etc. (Crop Management, 2020) have little impact on the effectiveness of Trichoderma spp. season-to season. Tillage practices have no impact on Trichoderma spp. colonization, they are not crop specific and colonize all crop irrespective of crop rotations and seed treatment is more effective in providing plant benefits (Siemering et al., 2016).
Can I use Trichoderma spp. and Arbuscular Mycoorhizal Fungi (AMF) inoculants at the same time?
Few research data suggest naturally co-existence of AMF and Trichoderma spp. in rhizosphere, work simultaneously during co-inoculation for increased crop yield by 37%. However, differ in concentrations of the inoculants may temporarily upset the work and benefit balance on the host plants (Siemering et al., 2016). The application of Trichoderma spp. favours the access of AMF to non-host rapeseed roots to produce significant increase in colonization, promote growth and stimulate systemic defense (Poveda et al., 2019). Work simultaneously with other species (Bacillus, and Trichoderma) to increase the availability of phosphorus and nitrogen fixation in soil to host plant (Schmidt et al., 2015) (Shivasakthi et al., 2014).
<< See Part 1 Continue to Part 3 >>
References:
- Mastouri, F., Björkman, T., & Harman, G. E. (2010). Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology, 100(11), 1213-1221.
- Zin, N. A., & Badaluddin, N. A. (2020). Biological functions of Trichoderma spp. for agriculture applications. Annals of Agricultural Sciences, 65(2), 168-178.
- Botero Ramírez, A. (2016). Effect of three Trichoderma species on clubroot disease in cabbage. Escuela de Posgrados. Universidad Nacional de Colombia, Masters Thesis.
- Suada, I. K. (2017). The potential of various indigenous Trichoderma spp. to suppress Plasmodiophora brassicae the pathogen of clubroot disease on cabbage. Biodiversitas Journal of Biological Diversity, 18(4), 1424-1429.
- Debao, X. T. Z. J. L. (1993). Antagonism of Trichoderma harzianum T82 and Trichoderma sp NF9 against soil-borne fungous pathogens [J]. Acta Phytopathologica Sinica, 1.
- Cheah, L. H., Veerakone, S., & Kent, G. (2000). Biological control of clubroot on cauliflower with Trichoderma and Streptomyces spp. New Zealand Plant Protection, 53, 18-21
- Upadhyay H., Kumar V., Kumar S. (2020). Chapter 6 – The effect of fungal metabolites on soil-borne pathogenic fungi. https://doi.org/10.1016/B978-0-12-821007-9.00006-1
- Singh, A., Shukla, N., Kabadwal, B. C., Tewari, A. K., & Kumar, J. (2018). Review on plant-Trichoderma-pathogen interaction. Int J Curr Microbiol Appl Sci, 7(2), 2382-2397.
- Sood, M., Kapoor, D., Kumar, V., Sheteiwy, M. S., Ramakrishnan, M., Landi, M., … & Sharma, A. (2020). Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants, 9(6), 762.
- Abbas, A., Jiang, D., & Fu, Y. (2017). Trichoderma spp. as antagonist of Rhizoctonia solani. Journal of Plant Pathology and Microbiology, 8(3).
- Poveda, J. (2020). Trichoderma parareesei favors the tolerance of rapeseed (Brassica napus L.) to salinity and drought due to a chorismate mutase. Agronomy, 10(1), 118.
- Bazghaleh, N., Prashar, P., Woo, S., & Vandenberg, A. (2020). Effects of Lentil Genotype on the Colonization of Beneficial Trichoderma Species and Biocontrol of Aphanomyces Root Rot. Microorganisms, 8(9), 1290.
- Tančić, S., Skrobonja, J., Lalošević, M., Jevtić, R., & Vidić, M. (2013). Impact of Trichoderma spp. on soybean seed germination and potential antagonistic effect on Sclerotinia sclerotiorum. Pesticidi i fitomedicina, 28(3), 181-185.
- Singh, B. N., Singh, A., Singh, G. S., & Dwivedi, P. (2015). Potential role of Trichoderma asperellum T42 strain in growth of pea plant for sustainable agriculture. J. Pure Appl. Microbiol, 9(2), 1069-1074.
- Kashem, M. A., Hossain, I., & Hasna, M. K. (2011). Use of Trichoderma in biological control of foot and root rot of lentil (Lens culinaris Medik). Int. J. Sustain. Crop Prod, 6(1), 29-35.
- Poveda, J., Hermosa, R., Monte, E., & Nicolás, C. (2019). Trichoderma harzianum favours the access of arbuscular mycorrhizal fungi to non-host Brassicaceae roots and increases plant productivity. Scientific reports, 9(1), 1-11.
- Siemering, G., Ruark, M., & Geven, A. (2016). The value of Trichoderma for crop production. University of Wisconsin–Extension, Cooperative Extension.
- Crop Management, (2020) https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/crops-and-irrigation/organic-crops/organic-crop-management-insect-management.
- Schmidt, J., Messmer, M., & Wilbois, K. P. (2015). Beneficial microorganisms for soybean (Glycine max (L.) Merr), with a focus on low root-zone temperatures. Plant and soil, 397(1-2), 411-445.
- Sivasakthi, S., Usharani, G., & Saranraj, P. (2014). Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: a review. African journal of agricultural research, 9(16), 1265-1277.