What is Bacillus spp.?
Bacillus spp. group of bacteria have own acronym as plant growth promoting rhizobacteria (PGPR), form a biofilm on plant root to promote plant growth and provide defensive action to fending off plant pathogens(George H, 2019). The population of B. subtilis, B. licheniformis and B. velezensis are the most common ones and found in healthy plants indicating efficient colonization in rhizosphere (Vinodkumar et al., 2018).
How can Bacillus spp help my crops?
Relief from environmental stresses
Bacillus spp. enhances pigments, hormones (SA, JA, and ABA) and enzymes (catalase, superoxide dismutase, peroxidase, ascorbate peroxidase and glutathione reductase) release to mitigate drought stress and increase water uptake rate. Up or down regulate the antioxidants (CAT, SOD, POD, APX, and GR) and drought responsible genes in host plants. Increase the population of bacterial colony on roots and stimulate root exudation to take up more water against drought damage. Regulate high-affinity transport (HKT1), hydraulic conductivity of roots, stomatal opening, turgor pressure maintenance, osmotic balance and modulate homeostasis (Na/K) to overcome drought stress conditions (Radhakrishnan et al., 2017).
Improved Nutrient efficiency
Bacillus spp. secrete enzymes and organic acids to facilitate the conversion of complex, inorganic phosphate into free, simple, available phosphate for root uptake to improve plant growth metabolisms. Bacillus spp. release ammonia from nitrogenous organic matter, fix atmospheric nitrogen and increase the available form of nitrogen in soil, which enhance plant growth and yield by delaying senescence. The iron-chelating properties of Bacillus spp. solubilize Fe3+ to Fe2+ for easy entry into plant cells to increase nutrient uptake. Induce the synthesis of plant-growth-promoting hormones such as indole-3-acetic acid (IAA), gibberellins, cytokinins, spermidines, to increase root/shoot cell division, elongation and secretion of 1-aminocyclopropane-1-carboxylate (ACC) deaminase to promote plant growth (Radhakrishnan et al., 2017).
Solutions to soils contaminated with heavy metals
Plants treated with Bacillus spp. solubilize, or convert toxic metals to non-toxic forms, reduce the toxic effects of these metals in soil and on plant growth. Enhances heavy metals (Cu, Zn, Cd, Cr and Pb) accumulation and distribution in plants growing in soil contaminated with heavy metals. The association of some Bacillus spp. regulate antioxidants in cells to inhibits oxidative stress damage and triggers plant growth-promoting substances to enable host plants to adapt to metal stress conditions (Radhakrishnan et al., 2017).
Plants immune system response
Bacillus spp. play a role of root colonizer, biocontrol agent, build secure zone, associated mechanism of acquired systemic resistance to increase crop yield under conditions of biotic and abiotic stress (Hashem et al., 2019) (Vinodkumar et al., 2017) as represented in figure 1.
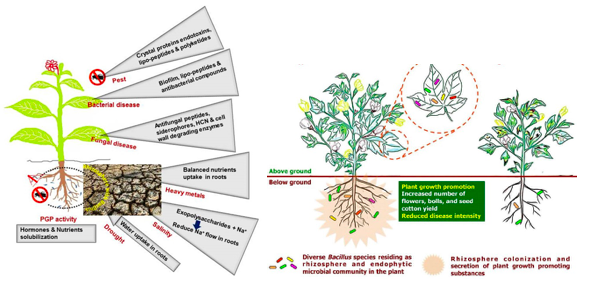
Bacillus spp. activate the immune system known as systemic acquired resistance (SAR) throughout the plant, to ramp-up the natural defence and inhibit soil-borne fungal pathogens (Rhizoctonia, Phytophthora, Fusarium, Botrytis, powdery mildew and Sclerotinia) and bacterial pathogens (Erwinia and Xanthomonas) (George H, 2019) (Radhakrishnan et al., 2017) (Vinodkumar et al., 2017). Various plants viz., carnations, cotton, turmeric, and bananas treated with Bacillus spp. demonstrated minimal percent (4.6%) of Sclerotinia disease incidence and delivered maximum plant growth yield (Vinodkumar et al., 2017) as represented in figure 2.
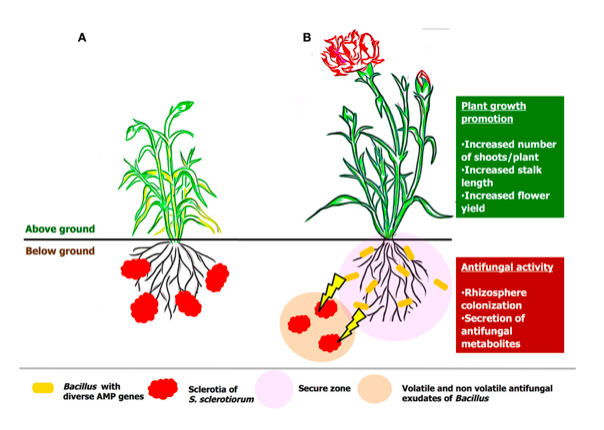
Figure 2: Hypothetical model on the mode of actions of Sclerotina before (A) and after treatment (B) of Bacillus spp. as represented by (Vinodkumar et al., 2017).
Plant disease management
Bacillus spp. exhibits both a direct (synthesis of many secondary metabolites, hormones, cell-wall-degrading enzymes, and antioxidants) and indirect (stimulation of acquired systemic resistance and expression of stress-response genes) biocontrol mechanism to suppress disease caused by pathogens (Vinodkumar et al., 2017). Species of Bacillus produce more than 24 types of antibiotics, antimicrobial metabolites, and other toxin compounds such as lipopeptides, Iturin A, fencygins, surfactin, macrolactin and bacillomycin etc. Lipopeptides interact with fungal cell membranes to create holes and to kill the fungal in the process. Iturin A and fencygins exhibit antifungal and antibacterial activity to inhibit spore generation and increased cell membrane permeability with formation of ion-conducting pores to damage and cause pest larval death(George H, 2019) (Radhakrishnan et al., 2017) (Vinodkumar et al., 2017).
In case of diseased plant, the pathogenic bacteria affect the defence system by decreasing the enzyme activity (phenylalanine ammonia-lyase (PAL), lipoxygenase etc.). However, with the application of Bacillus spp. these defence enzyme and antioxidant enzymes activities are accelerated, triggering the biosynthesis of polyphenol compounds (lignin, flavonoids and phenylpropanoids) to develop plant resistance against environmental stimuli(Radhakrishnan et al., 2017). Bacillus spp. release antiviral compounds to fight against viral pathogens, considered as second largest group of plant disease after fungi. In addition, trigger induced systemic resistance (ISR), biofilm formation, surfactin production and increase the expression of disease-resistant signaling genes, defence genes and cell wall expansion genes in plants.
Altogether, inhibit viral coat protein synthesis, prevent viral damage, and provide enhanced plant growth against cucumber and tobacco mosaic virus infection in plants. In case of root-knot nematodes, Bacillus spp. prevent the infections of nematode populations, inhibit the growth, develop resistance by reducing gall, egg masses in plants and release crystal proteins responsible for nematicidal activity against nematodes(Radhakrishnan et al., 2017). Exploitation of Bacillus spp. with multifaceted action and with diverse anti-microbial genes will together serve an alternative method for the management and control of fungal, bacterial, and viral diseases in crop plants.
Should I inoculate my crops with Bacillus spp.?
Increased crop yield
The application of Bacillus spp. on soybean was carried out in a conventional system under sprinkler irrigation, resulted in increased yield (15-25%) due to increased accumulation of storage proteins and seed vigor (Tavanti et al., 2020), decreased root rot occurrence by (43-63%), increased plant height (9-18%), root dry weight (8-19%) and altogether suggesting effective alternatives to fungicides (Zhang et al., 2009). In case of cowpea and mash bean plants, the Bacillus spp. application as seed dressing and soil drenching resulted in significant increase in germination (70-100%), length (2-7%) and weight (2-5%) of shoot and root and controlled the infection of soil borne root-infecting fungi (Dawar et al., 2010). The disease management of vascular wilt on lentils using Bacillus spp. delivered highest inhibition of 36%, improved growth and increased dry weight of lentil plants (El‐Hassan et al., 2006). The effect of Bacillus spp. on canola plant reduced clubroot severity by 62-83% due to upregulation of defense response gene by 2.2-23-fold relative to control plants (Lahlali et al., 2013) and in small-scale trial significantly controlled the damping-off and occurrence of fungal disease on alfalfa seeds (Handelsman et al., 1990).
Will my crop management practice impact Bacillus spp growth?
Crop management practise such as crop rotations, tillage, seed variety, seeding rate, sanitation, summer-fallow, trap strips, soil fertility, intercrops, biological control, pest, and insect management etc. (Crop Management, 2020) have little impact on the effectiveness of AMF season-to season. The application of Bacillus as PGPRs introduced in agricultural systems will increase the resistance towards biotic and abiotic stresses to produce sustainable, environmentally-friendly management tools.
Can I use Bacillus spp. with other inoculants at the same time?
Bacillus spp. can be used simultaneously along with biocontrol inoculants, which posses the property of forming plant growth promoting rhizobacteria (PGPRs) such as Bacillus, Pseudomonas, and Trichoderma (Panpatte et al., 2016). The Bacillus spp. plays a major role in PGPRs to deliver benefits across plant growth promotion and suppression of diseases (Negi et al., 2008) and work simultaneously with other species (Pseudomonas, and Trichoderma) to increase the availability of phosphorus and nitrogen fixation in soil to host plant (Schmidt et al., 2015) (Shivasakthi et al., 2014). Soils inoculated with the combination of Bacillus spp. and Trichoderma improved the efficacy of an antagonists and reduced the occurrence of infections to seeds, this effect increased with increased dose (Dawar et al., 2010) and consortial application with other beneficial microbes resulted in reduced cotton leaf curl occurrence and resistance against cucumber mosaic virus (Vinodkumar et al., 2018).
References:
- George H, (2019) Controlling plant pathogens with the biofungicide Bacillus subtilis, link: https://gardenerspath.com/how-to/organic/bacillus-subtilis/
- Radhakrishnan, R., Hashem, A., & Abd_Allah, E. F. (2017). Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments. Frontiers in physiology, 8, 667.
- Hashem, A., Tabassum, B., & Abd_Allah, E. F. (2019). Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi journal of biological sciences, 26(6), 1291-1297.
- Vinodkumar, S., Nakkeeran, S., Renukadevi, P., & Malathi, V. G. (2017). Biocontrol potentials of antimicrobial peptide producing Bacillus species: multifaceted antagonists for the management of stem rot of carnation caused by Sclerotinia sclerotiorum. Frontiers in microbiology, 8, 446.
- Vinodkumar, S., Nakkeeran, S., Renukadevi, P., & Mohankumar, S. (2018). Diversity and antiviral potential of rhizospheric and endophytic Bacillus species and phyto-antiviral principles against tobacco streak virus in cotton. Agriculture, Ecosystems & Environment, 267, 42-51.
- Tavanti, T. R., Tavanti, R. F., Galindo, F. S., Simões, I., Dameto, L. S., & Sá, M. E. D. (2020). Yield and quality of soybean seeds inoculated with Bacillus subtilis strains. Revista Brasileira de Engenharia Agrícola e Ambiental, 24(1), 65-71.
- Zhang, J. X., Xue, A. G., & Tambong, J. T. (2009). Evaluation of seed and soil treatments with novel Bacillus subtilis strains for control of soybean root rot caused by Fusarium oxysporum and F. graminearum. Plant disease, 93(12), 1317-1323.
- Dawar, S., Wahab, S., Tariq, M., & Zaki, M. J. (2010). Application of Bacillus species in the control of root rot diseases of crop plants. Archives of Phytopathology and Plant Protection, 43(4), 412-418.
- El‐Hassan, S. A., & Gowen, S. R. (2006). Formulation and delivery of the bacterial antagonist Bacillus subtilis for management of lentil vascular wilt caused by Fusarium oxysporum f. sp. lentis. Journal of Phytopathology, 154(3), 148-155.
- Lahlali, R., Peng, G., Gossen, B. D., McGregor, L., Yu, F. Q., Hynes, R. K., … & Boyetchko, S. M. (2013). Evidence that the biofungicide Serenade (Bacillus subtilis) suppresses clubroot on canola via antibiosis and induced host resistance. Phytopathology, 103(3), 245-254.
- Handelsman, J., Raffel, S., Mester, E. H., Wunderlich, L., & Grau, C. R. (1990). Biological control of damping-off of alfalfa seedlings with Bacillus cereus UW85. Applied and environmental microbiology, 56(3), 713-718.
- Crop Management, (2020) https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/crops-and-irrigation/organic-crops/organic-crop-management-insect-management.
- Schmidt, J., Messmer, M., & Wilbois, K. P. (2015). Beneficial microorganisms for soybean (Glycine max (L.) Merr), with a focus on low root-zone temperatures. Plant and soil, 397(1-2), 411-445.
- Sivasakthi, S., Usharani, G., & Saranraj, P. (2014). Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: a review. African journal of agricultural research, 9(16), 1265-1277.