What is Arbuscular Mycorrhizal Fungi?
Arbuscular Mycorrhizal Fungi (AMF) also known as “root allies” is a major group of root mutualistic endophytic soil microorganisms, develops mutual symbiotic association with roots of most terrestrial plant species including grass, clover, legume tree, etc. These are obligate biotrophs dependent on the host root tissue for carbohydrate supply and to complete their asexual life cycle (Pozo et al., 2013) (Yang et al., 2016). AMF colonize plant root through soil-based propagules (such as spores, fragments of mycorrhizal roots and extraradical hyphae) to form “arbuscules” characteristic tree like structures, where most of nutrient exchange occurs (Pozo et al., 2013).
How can AMF help my crops?
Relief from environmental stresses
The symbiosis of AMF promotes plant growth, increases the immobile soil minerals and nutrient uptake rate, enhances resistance to drought and stress tolerance, protects plants against soilborne fungal and bacterial pathogens, nematodes, insects, and induces resistance against shoot pathogens (Pozo et al., 2013) (Yang et al., 2016) (Jacott et al., 2017) (Evelin et al., 2019) as represented in figure 1. The symbiotic relation regulates different physio-biochemical process such as enhanced osmotic regulation, stomatal regulation, increased accumulation of proline and glutathione, altogether improve root size, leaf area index and biomass against severe drought and stress conditions (Begum et al., 2017).
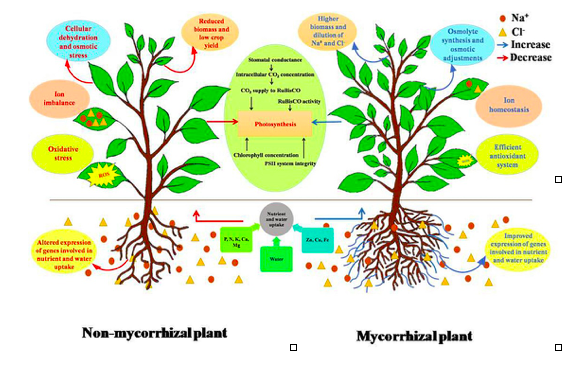
Figure 1: Differential responses of non-mycorrhizal and mycorrhizal plant as represented by (Evelin et al., 2019).
Improved Nutrient efficiency
The ecological, physiological, and molecular basis of AMF functioning and their implications in enabling the plant improvement through biotic (e.g., pathogen and herbivore attack), abiotic stress (e.g., drought, salinity, heavy metals toxicity, or presence of organic pollutants) and improves soil composition with formation of hydro-stable aggregates essential for good soil tilth (Pozo et al., 2013). Under salinity conditions, AMF inoculated plants showed enhanced leaf water potential, water utilization efficiency, increased photosynthetic rate, improved gas exchange traits, adaptation in ionic relations and higher chlorophyll content in comparison to nonmycorrhizal plants under saline growth conditions (Begum et al., 2017).
Biotic and Abiotic conditions
The extraradical mycelium forms extensive mycelial network for the acquisition of mineral nutrients (phosphate, iron, and ammonia), which are in ionic form with poor mobility or present at very low concentrations in soil environment (Pozo et al., 2013). The AMF symbiosis with host plant extends the network of roots beyond the depletion zone as represented in figure 2, overcome the diffusion limitation, cover a larger area of soil, and enable more efficient up-take rate of phosphate by 70% (Jacott et al., 2017).
Solutions to soils contaminated with heavy metals
AMF-colonized plants have positive effect on abiotic stress tolerance due to plant water relation, accumulation of soluble sugars in roots, improved nutrient acquisition, reduced symptoms of arsenic toxicity, low accumulation of heavy metals and minimum host sulphur starvation response (Jacott et al., 2017). In addition, AMF supports the establishment and growth of plants in soil contaminated with heavy metals. In the presence of AMF, heavy metals are accumulated by high cation-exchange capacity, immobilized in the hyphae, fix metals in vacuole/cell walls, and chelate the metals with other substances to dilute the metal concentrations in the soil (Begum et al., 2017) (Parihar et al., 2020).
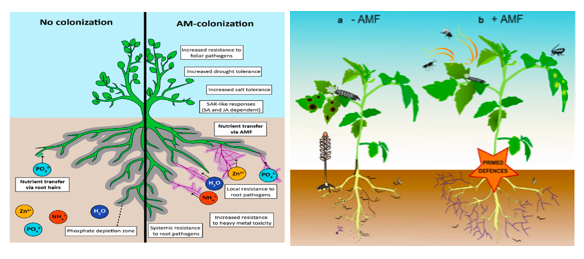
Figure 2: Positive effects and plant disease management by Arbuscular Mycorrhizal Fungi as represented by (Jacott et al., 2017) and (Pozo et al., 2013).
Plants immune system response
AMF modulates the host plants immune system and Induced Systemic Resistance (ISR), reprogram plant gene expression, alter in secretion of metabolites (primary and secondary) related to plant defense, trigger plant defense response and pre-condition of priming alert state for faster and stronger response to any pathogen and pests attack (Pozo et al., 2013). In case of parasitic plants and weeds, the AMF down regulates the level of strigolactones (SLs) involved in the germination rate of weed seeds, decrease the occurrence and the damage of root parasitic plants, and suppress the growth of aggressive agricultural weeds (Pozo et al., 2013).
In case of disease resistance, AMF plants deliver increased nutrient status, direct competition with root space and nutrients causing reduction in root pathogens, induce long-lasting systemic acquired resistance (SAR), increased production of defence-compounds (phenols, glucanase and chitinolytic enzymes) and upregulation of systemic priming isoforms to challenge pathogen diseases caused by (Aphanomyces euteiches, Pythium ultimum, Phytophthora parasitica, Magnaporthe oryzae, Alternaria solani, Botrytis cinerea etc.) (Jacott et al., 2017).
Plant disease management
AMF protection against soil-borne pathogens includes series of events such as alteration of root morphology to inhibit pathogen infection, antagonize possible root pathogens, enhanced resistance due to improvement in plant defence, root protection, formation of clear protective effect against pests, reduce the damage caused by soil-borne pathogens, direct compete for space and nutrition’s with pathogens, and detrimental response on parasitic nematodes (Pozo et al., 2013) (Posta et al., 2020) as represented in figure 3.
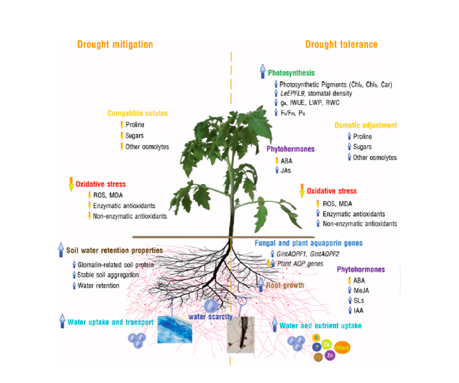
In case of leaf pathogens, AMF responds with two main plant-mediated mechanisms. The first one involves the activation of the host plant defense response. In the second mechanism, AMF involves a plant to overcome herbivore attach or quickly recover from the pathogen attach by altering source-sink relation and improving the nutritional status of the host plant (Pozo et al., 2013) (Posta et al., 2020).
Should I inoculate my crops with AMF?
Increased crop yield
Application of AMF altered plant growth, yield and quality: resulted in significant increases in shoot dry weight (by 128–242%) and root dry weight (185–328%) in French lavender (Lavandula dentata) plants, in tomato increased shoot dry weight under drought conditions, accelerates flowering and fruit development, and tomato yield (by 51–71%) in plants subjected to 50% water supply (Posta et al., 2020). Lentil treated with AMF displayed increased seed germination by 9-11%, root colonization by 40%, and increased phosphorus uptake efficiency. In case of legumes (lentils, soybean, and beans) AMF treatment significantly increased plant dry weight, N and P content and seed yield (Rahman et al., 2017, Zarei et al., 2006, El-Din et al., 1988, El-Din et al., 2019). Canola treated with AMF, inhibited non-host plants, and promoted the plant growth indirectly by increased nutrients (N, P, K, and S) mobilization (Dąbrowska et al., 2014). Improves nutritional value through enhanced uptake of micronutrients in plants like wheat, maize, linseed, and sunflower (Ryan et al., 2002) improved growth and plant nutrient response in plant species, including cereals, legumes, and vegetables (Tran et al., 2019).
Will my crop management practice impact AMF growth?
Crop management practise such as crop rotations, tillage, seed variety, seeding rate, sanitation, summerfallow, trap strips, soil fertility, intercrops, biological control, pest, and insect management etc. (Crop Management, 2020) have little impact on the effectiveness of AMF season-to season (Berrutietal et al.,2020). Use of mechanical and chemical treatments in the presence of the AMF treated field resulted in lower AMF hyphal lengths, disrupt communities in terms of species diversity, suggesting AMF depended on biological process in organic farming systems (Bilalis et al., 2011). The conventional breeding programs need to consider the requirement characteristics of soil to facilitate the colonization of AMF to support host plants to stimulate against drought stress and higher crop tolerance (Posta et al., 2020).
Can I use AMF and Trichoderma spp. inoculants at the same time?
Combination of AMF with other useful microbes such as Trichoderma spp. or Plant growth-promoting bacteria (PGPR) results into synergetic effects on plant tolerance over drought and stressful conditions (Posta et al., 2020). The research data suggest naturally co-existence of AMF and Trichoderma spp. in rhizosphere, work simultaneously during co-inoculation for increased crop yield by 37%. However, differ in concentrations of the inoculants may temporarily upset the work and benefit balance on the host plants (Siemering et al., 2016). Presence of Trichoderma spp. favours the access of AMF to non-host rapeseed roots to produce significant increase in colonization, promote growth and stimulate systemic defense (Poveda et al., 2019).
References:
- Pozo, M. J., Jung, S. C., Martínez-Medina, A., López-Ráez, J. A., Azcón-Aguilar, C., & Barea, J. M. (2013). Root allies: arbuscular mycorrhizal fungi help plants to cope with biotic stresses. In Symbiotic endophytes (pp. 289-307). Springer, Berlin, Heidelberg.
- Yang, Y., Liang, Y., Han, X., Chiu, T. Y., Ghosh, A., Chen, H., & Tang, M. (2016). The roles of arbuscular mycorrhizal fungi (AMF) in phytoremediation and tree-herb interactions in Pb contaminated soil. Scientific reports, 6, 20469.
- Jacott, C. N., Murray, J. D., & Ridout, C. J. (2017). Trade-offs in arbuscular mycorrhizal symbiosis: disease resistance, growth responses and perspectives for crop breeding. Agronomy, 7(4), 75.
- Begum, N., Qin, C., Ahanger, M. A., Raza, S., Khan, M. I., Ahmed, N., … & Zhang, L. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Frontiers in plant science, 10, 1068.
- Posta, K., & Duc, N. H. (2020). Benefits of arbuscular mycorrhizal fungi application to crop production under water scarcity. Drought-Detection and Solutions, 25-37.
- Parihar, M., Chitara, M., Khati, P., Kumari, A., Mishra, P. K., Rakshit, A., … & Bisht, J. K. (2020). Arbuscular Mycorrhizal Fungi: Abundance, Interaction with Plants and Potential Biological Applications. In Advances in Plant Microbiome and Sustainable Agriculture (pp. 105-143). Springer, Singapore.
- Evelin, H., Devi, T. S., Gupta, S., & Kapoor, R. (2019). Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges. Frontiers in Plant Science, 10, 470.
- Rahman, M., Ali, M. E., Alam, F., Banu, M. B., Anik, M. F. A., & Bhuiyan, M. A. H. (2017). Effect of Arbuscular Mycorrhizal Fungi on Germination, Nodulation and Sporulation of Lentil (Lens culinaris) at Different NaCl Levels. Bangladesh Journal of Microbiology, 34(2), 73-81.
- Zarei, M., Saleh-Rastin, N., Alikhani, H. A., & Aliasgharzadeh, N. (2006). Responses of lentil to co-inoculation with phosphate-solubilizing rhizobial strains and arbuscular mycorrhizal fungi. Journal of plant nutrition, 29(8), 1509-1522.
- El-Din, S. B., & Moawad, H. (1988). Enhancement of nitrogen fixation in lentil, faba bean, and soybean by dual inoculation with Rhizobia and mycorrhizae. Plant and Soil, 108(1), 117-123.
- Alam, M. Z., Hoque, M. A., Ahammed, G. J., & Carpenter-Boggs, L. (2019). Arbuscular mycorrhizal fungi reduce arsenic uptake and improve plant growth in Lens culinaris. PloS one, 14(5), e0211441.
- Dąbrowska, G., Baum, C., Trejgell, A., & Hrynkiewicz, K. (2014). Impact of arbuscular mycorrhizal fungi on the growth and expression of gene encoding stress protein–metallothionein BnMT2 in the non‐host crop Brassica napus L. Journal of Plant Nutrition and Soil Science, 177(3), 459-467.
- Ryan, M. H., & Graham, J. H. (2002). Is there a role for arbuscular mycorrhizal fungi in production agriculture?. Plant and soil, 244(1-2), 263-271.
- Tran, B. T., Watts-Williams, S. J., & Cavagnaro, T. R. (2019). Impact of an arbuscular mycorrhizal fungus on the growth and nutrition of fifteen crop and pasture plant species. Functional Plant Biology, 46(8), 732-742.
- Crop Management, (2020) https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/crops-and-irrigation/organic-crops/organic-crop-management-insect-management.
- Siemering, G., Ruark, M., & Geven, A. (2016). The value of Trichoderma for crop production. University of Wisconsin–Extension, Cooperative Extension.
- Poveda, J., Hermosa, R., Monte, E., & Nicolás, C. (2019). Trichoderma harzianum favours the access of arbuscular mycorrhizal fungi to non-host Brassicaceae roots and increases plant productivity. Scientific reports, 9(1), 1-11.
- Bilalis, D., Karkanis, A., Konstantas, A., Patsiali, S., & Triantafyllidis, V. (2011). Arbuscular mycorrhizal fungi: a blessing or a curse for weed management in organic olive crops?. Australian Journal of Crop Science, 5(7), 858.
- Berruti, A., Lumini, E., Balestrini, R., & Bianciotto, V. (2016). Arbuscular mycorrhizal fungi as natural biofertilizers: let’s benefit from past successes. Frontiers in microbiology, 6, 1559.