What is Pseudomonas fluorescens?
Pseudomonas spp. are bacterial species ubiquitous in agricultural soils, well adapted to growing in the rhizosphere and well suited as biocontrol and growth-promoting agents (Weller et al., 2007). Pseudomonas spp. rapidly utilize root and seed exudates, multiply in rhizosphere and spermoshphere environment and in the interior of the plants, produce a wide spectrum of bioactive metabolites (such as antibiotics, siderophores, volatiles, and growth-promoting substances); adapt to environmental stresses and compete aggressively with pathogens (Weller et al., 2007) (Panpatte et al., 2016).
How can Pseudomonas spp. help my crops?
Relief from environmental stresses
Colonies of Pseudomonas spp. are aggressive to persist throughout the growing season, limit the amount of minerals and nutrient availability to pathogens for growth, pre-empt and compete with pathogens for the favored sites on the root, and improve plant growth by suppressing major (root or vascular diseases) and minor (parasites or saprophytes) plant pathogens (Weller et al., 2007). Pseudomonas spp. solubilize phosphorous in soil, release chelating agent called siderophores for iron absorption, nitrogen fixing ability, increase in elements availability to plants to enhance plant growth (Shivasakthi et al., 2014) (Panpatte et al., 2016).
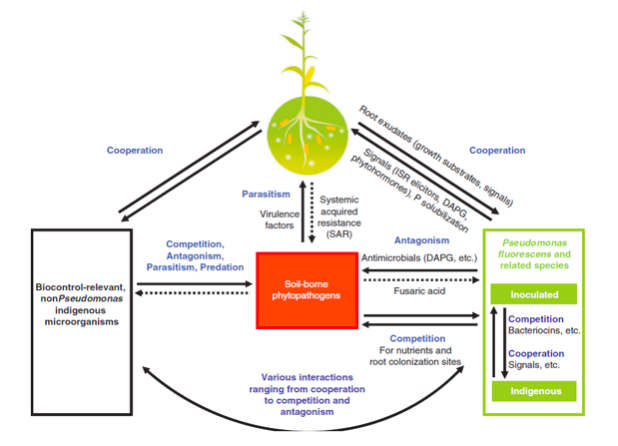
Figure 1: Plant disease management by Pseudomonas spp. as represented by (Couillerot et al., 2008)
Plants immune system response
Pseudomonas spp. demonstrate dynamics of root colonization depicting high microbial diversity, density, synchronized metabolic activity, formidable biological buffering capacity that not only protect from foreign pathogens but also from the introduced pathogens (Weller et al., 2007). During the plant growth application, Pseudomonas spp. produce a potent antibiotic resulting in control and suppression of bacterial disease fire blight even in the areas with streptomycin-resistant populations of the pathogen and provide moderate to excellent efficacy (Couillerot et al., 2008) as represented in figure 1. Pseudomonas spp. produce antimicrobial and metabolites, such as 2,4-diacetylphloroglucinol (DAPG), phenazines, hydrogen cyanide and surfactants, which have received extensive attention and efficacy rate to inhibit various phytopathogens. Pseudomonas spp. also produce extracellular lytic enzymes for protection of plant species and induce an ISR response in plants, and to make plants efficient in fighting back against pathogens (Couillerot et al., 2008).
Plant disease management
The secretion of anti-fungal metabolites by Pseudomonas spp. effectively inhibited mycelial growth by 62-85% and at increased metabolite concentration of 5% completely inhibited the growth of fungal pathogens such as Rhizoctonia bataticola, Fusaricum oxysporum, Xanthomonas oryzae, Rhizoctonia solani (bacterial leaf blight (BB) and sheath blight (ShB) pathogens of rice) (Shivasakthi et al., 2014). Pseudomonas spp. shows high efficacy in controlling the pathogen infections in immature pear fruit, flowers, and whole plants (Pujol et al., 2006). Pseudomonas spp. are also applied in the packinghouses to prevent postharvest fungal disease during storage of citrus, pome, stone fruits, and potatoes (Stockwell et al., 2007).
Should I inoculate my crops with Pseudomonas spp.?
Increased crop yield
The application of Pseudomonas spp. as seed inoculants on crop plants rapidly colonize plant roots, promote growth, enhance crop yield by direct and indirect mechanisms, increase in fresh and dry plant masses resulting in increased yield by 44% (Shivasakthi et al., 2014) (Panpatte et al., 2016). Field experiments on chickpea treated with Bacillus and Pseudomonas spp. delivered maximum significant increase in nodule number, dry weight of nodule, root and shoot and due to additional accumulation of phosphorus, iron, and plant hormones like indole-3-acetic acid (IAA) (Verma et al., 2010).
In presence of Pseudomonas spp. the canola plants delivered increased uptake of phosphorous, improved root (61-65%) and shoot (33-47%) elongation, and promoted plant growth under the gnotobiotic conditions (Lifshitz et al., 1987). In case of soyabean, the Pseudomonas spp. treatment resulted in increased shoot (14-26%) and yield (9-36%) (Schmidt et al., 2015), in alfalfa increased the alginate biosynthesis by 3-fold and decreased the disease infection frequency by 50% (Silo-Suh et al., 2002). In chickpeas increased the biochemical properties of the crop under the soil contaminated with chromium (Oves et al., 2013) and reduction in plant mortality by (63.3-100%) (Negi et al., 2008). Application of Pseudomonas spp. on lentils cultivated in soil depleted with nutrients, resulted in 85% of nodule formation, higher nodule occupancy and delivered increased nitrogen fixation (Sepúlveda-Caamaño et al., 2018) and seed treatment with foliar applications improved yield in lentils (Erdemci et al., 2020).
Will my crop management practice impact Pseudomonas spp. growth?
Crop management practise such as crop rotations, tillage, seed variety, seeding rate, sanitation, summerfallow, trap strips, soil fertility, intercrops, biological control, pest, and insect management etc. (Crop Management, 2020) have little impact on the effectiveness of Pseudomonas spp. season-to season. The Pseudomonas spp. communities are affected long term by plant species and genotypes and through short-term effects during the lifespan of single plant (Schmidt et al., 2015). The current findings on the Pseudomonas spp. are gaining broader incorporation into sustainable agriculture strategies for the crop management against soil pathogens and post-harvest disease (Weller et al., 2007).
Can I use Pseudomonas spp. with other inoculants at the same time?
Pseudomonas spp. can be used simultaneously along with biocontrol inoculants, which posses the property of forming plant growth promoting rhizobacteria (PGPRs) such as Bacillus, Pseudomonas, and Trichoderma (Panpatte et al., 2016). The Pseudomonas spp. plays a major role in PGPRs to deliver benefits across plant growth promotion and suppression of diseases (Negi et al., 2008) and work simultaneously with other species (Bacillus, and Trichoderma) to increase the availability of phosphorus and nitrogen fixation in soil to host plant (Schmidt et al., 2015) (Shivasakthi et al., 2014).
References:
- Pujol, M., Badosa, E., Manceau, C., & Montesinos, E. (2006). Assessment of the environmental fate of the biological control agent of fire blight, Pseudomonas fluorescens EPS62e, on apple by culture and real-time PCR methods. Applied and Environmental Microbiology, 72(4), 2421-2427.
- Weller, D. M. (2007). Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology, 97(2), 250-256.
- Stockwell, V. O., & Stack, J. P. (2007). Using Pseudomonas spp. for integrated biological control. Phytopathology, 97(2), 244-249.
- Couillerot, O., Prigent‐Combaret, C., Caballero‐Mellado, J., & Moënne‐Loccoz, Y. (2009). Pseudomonas fluorescens and closely related fluorescent pseudomonads as biocontrol agents of soil‐borne phytopathogens. Letters in applied microbiology, 48(5), 505-512.
- Sivasakthi, S., Usharani, G., & Saranraj, P. (2014). Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: a review. African journal of agricultural research, 9(16), 1265-1277.
- Panpatte, D. G., Jhala, Y. K., Shelat, H. N., & Vyas, R. V. (2016). Pseudomonas fluorescens: a promising biocontrol agent and PGPR for sustainable agriculture. In Microbial inoculants in sustainable agricultural productivity (pp. 257-270). Springer, New Delhi.
- Verma, J. P., Yadav, J., & Tiwari, K. N. (2010). Application of Rhizobium sp. BHURC01 and plant growth promoting rhizobactria on nodulation, plant biomass and yields of chickpea (Cicer arietinum L.). International Journal of Agricultural Research, 5(3), 148-156.
- Lifshitz, R., Kloepper, J. W., Kozlowski, M., Simonson, C., Carlson, J., Tipping, E. M., & Zaleska, I. (1987). Growth promotion of canola (rapeseed) seedlings by a strain of Pseudomonas putida under gnotobiotic conditions. Canadian Journal of Microbiology, 33(5), 390-395.
- Sepúlveda-Caamaño, M., Gerding, M., Vargas, M., Moya-Elizondo, E., Oyarzúa, P., & Campos, J. (2018). Lentil (Lens culinaris L.) growth promoting rhizobacteria and their effect on nodulation in co-inoculation with rhizobia. Archives of Agronomy and Soil Science, 64(2), 244-256.
- Erdemci, İ. (2020). Effect of Pseudomonas Fluorescent Rhizobacteria on Growth and Seed Quality in Lentil (Lens Culinaris Medik.). Communications in Soil Science and Plant Analysis, 51(14), 1852-1858.
- Schmidt, J., Messmer, M., & Wilbois, K. P. (2015). Beneficial microorganisms for soybean (Glycine max (L.) Merr), with a focus on low root-zone temperatures. Plant and soil, 397(1-2), 411-445.
- Silo-Suh, L., Suh, S. J., Sokol, P. A., & Ohman, D. E. (2002). A simple alfalfa seedling infection model for Pseudomonas aeruginosa strains associated with cystic fibrosis shows AlgT (sigma-22) and RhlR contribute to pathogenesis. Proceedings of the National Academy of Sciences, 99(24), 15699-15704.
- Oves, M., Khan, M. S., & Zaidi, A. (2013). Chromium reducing and plant growth promoting novel strain Pseudomonas aeruginosa OSG41 enhance chickpea growth in chromium amended soils. European journal of soil biology, 56, 72-83.
- Negi, Y. K., Garg, S. K., & Kumar, J. (2008). Plant growth promoting and biocontrol activities of cold-tolerant Pseudomonas fluorescens isolates against root rot in pea. Indian Phytopathology, 61(4), 461.
- Crop Management, (2020) https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/crops-and-irrigation/organic-crops/organic-crop-management-insect-management
About Author: VINAYAK PACHAPUR, PhD

Plant growth mechanism are capable to overcome stress conditions (biotic and abiotic) and develop defense system to resist disease and pathogen occurrence. However, plants need symbiotic and synchronization of beneficial microbes to have stronger growth mechanism and defense system for better yield. To support this approach, Vinayak and CAI team embarked on developing bio-based formulations to boost the consortia between plant and beneficial microbes for better agriculture management and sustainable tool to higher yields.